Electric vehicles (EVs) are becoming more popular as a green and sustainable alternative to fossil fuel-powered cars. However, one of the main challenges that EV drivers face is the long charging time of the batteries, which can take hours to fully replenish. This can cause “range anxiety”, the fear of running out of power before reaching the destination or a charging station.
To address this problem, a team of engineers from Cornell University has developed a new lithium battery that can charge in as little as five minutes, faster than any such battery on the market. The breakthrough could revolutionize the EV industry and make electric transportation more convenient and accessible.
Table of Contents
How does the new battery work?
The new battery is based on a novel design principle that allows the metal ions at the anode, the negative electrode of the battery, to move freely and quickly in the solid state, without forming harmful deposits or dendrites that can degrade the battery’s performance and safety.
The researchers used indium, a soft metal that is mostly used for making touch-screen displays and solar panels, as the anode material. Indium has two crucial characteristics that make it ideal for fast-charging batteries: a very low migration energy barrier, which sets the rate at which ions diffuse in the solid state; and a modest exchange current density, which is related to the rate at which ions are reduced in the anode.
The combination of these qualities results in rapid diffusion and slow surface reaction kinetics, which are essential for fast charging and long-duration storage. The researchers found that after fast charging their new lithium battery, the indium anode had a smooth lithium electrodeposition, whereas other anode materials can grow dendrites that impact the battery’s performance.
What are the benefits of the new battery?

The new battery has several advantages over conventional lithium-ion batteries, which are currently the most widely used type of batteries for EVs and smartphones. The new battery can:
- Charge in under five minutes, compared to hours for conventional batteries.
- Maintain stable performance over thousands of cycles of charging and discharging, without losing capacity or efficiency.
- Reduce the size and cost of the battery pack, as less battery material is needed to achieve the same range and power output.
- Enhance the safety and reliability of the battery, as there is no risk of short circuits or fires caused by dendrite formation.
The new battery could also enable wireless induction charging on roadways, which would allow EVs to charge while driving, eliminating the need for charging stations and plugs.
What are the challenges and limitations of the new battery?
The new battery is still in the early stages of development and has not been tested in real-world conditions or scaled up for commercial production. The researchers acknowledge that there are some challenges and limitations that need to be overcome before the new battery can be widely adopted. Some of these are:
- The availability and cost of indium, which is a rare and expensive metal that is mostly mined in China and has limited reserves.
- The environmental impact of indium mining and processing, which can cause water pollution, soil erosion, and greenhouse gas emissions.
- The compatibility and integration of the new battery with existing EV systems and infrastructure, which may require modifications or adaptations.
- The regulatory and ethical issues related to the use and disposal of the new battery, which may pose potential risks to human health and the environment.
What are the future prospects of the new battery?
The new battery is a promising innovation that could transform the EV industry and pave the way for a cleaner and more efficient transportation system. The researchers hope that their work will inspire further research and development of fast-charging batteries and other energy storage technologies.
The new battery is also an example of how engineering can solve real-world problems and create positive social and environmental impacts. The researchers hope that their work will raise awareness and interest in engineering among the public and the next generation of engineers.
The new battery is not yet available for commercial use, but the researchers are optimistic that it will be in the near future. They are currently working on improving the performance and scalability of the new battery, as well as exploring other potential applications and markets.
The new battery is a result of a collaborative effort between Cornell Engineering, the Cornell Center for Materials Research, and the Cornell High Energy Synchrotron Source. The research was funded by the National Science Foundation and the U.S. Department of Energy.
What are the other types of batteries?
There are many types of batteries that have different characteristics and applications. Some of the common types of batteries are:
- Alkaline batteries: These are primary batteries that use zinc and manganese dioxide as electrodes and potassium hydroxide as electrolyte. They are widely used in household devices such as flashlights, toys, and remote controls. They have a high energy density, long shelf life, and low cost, but they are not rechargeable and can leak or corrode over time.
- Nickel-cadmium batteries (Ni-Cd): These are secondary batteries that use nickel and cadmium as electrodes and potassium hydroxide as electrolyte. They are used in power tools, medical equipment, and emergency lighting. They have a high power output, fast charging, and good performance in low temperatures, but they suffer from memory effect, self-discharge, and environmental issues.
- Nickel-metal hydride batteries (Ni-MH): These are secondary batteries that use nickel and a metal alloy as electrodes and potassium hydroxide as electrolyte. They are used in digital cameras, laptops, and hybrid vehicles. They have a higher energy density, longer cycle life, and less environmental impact than Ni-Cd batteries, but they have a lower power output, slower charging, and higher self-discharge.
- Lithium-ion batteries (Li-ion): These are secondary batteries that use lithium and a metal oxide as electrodes and a liquid or polymer electrolyte. They are used in smartphones, tablets, laptops, and electric vehicles. They have the highest energy density, longest shelf life, and no memory effect among common batteries, but they are expensive, sensitive to temperature, and prone to overheating or catching fire.
- Zinc-carbon batteries: These are primary batteries that use zinc and carbon as electrodes and ammonium chloride or zinc chloride as electrolyte. They are used in low-drain devices such as clocks, radios, and smoke detectors. They are cheap, simple, and widely available, but they have a low energy density, short shelf life, and poor performance in high-drain or low-temperature conditions.
- Lithium-sulfur batteries (Li-S): These are secondary batteries that use lithium and sulfur as electrodes and a liquid electrolyte. They are still in the research and development stage, but they have the potential to be used in electric vehicles, drones, and satellites. They have a higher energy density, lower cost, and lower environmental impact than Li-ion batteries, but they have a low power output, short cycle life, and stability issues.
The History of Battery
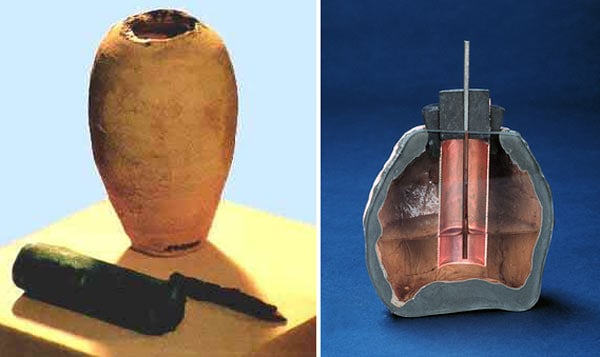
- The earliest evidence of a battery-like device dates back to around 250 BC, when the ancient Mesopotamians and Indians used clay jars filled with vinegar or lemon juice and metal rods to produce electric currents. These devices are known as the Baghdad Battery and the Agastya Samhita Battery, respectively.
- The term “battery” was first used by Benjamin Franklin in 1749, when he experimented with a series of linked Leyden jars, which are glass containers that can store electric charge.
- The first true chemical battery was invented by Alessandro Volta in 1800, when he stacked discs of copper and zinc separated by cloth soaked in salt water. This device, called the voltaic pile, generated a steady electric current and enabled many scientific discoveries.
- The first practical battery was developed by John Daniell in 1836, when he improved the voltaic pile by using a copper pot filled with copper sulfate solution and a zinc rod immersed in sulfuric acid. This battery, called the Daniell cell, reduced the corrosion of the electrodes and increased the voltage.
- The first rechargeable battery was invented by Gaston Planté in 1859, when he used lead plates and sulfuric acid to create a battery that could be recharged by reversing the current. This battery, called the lead-acid battery, is still widely used today for starting cars and other applications.
- The first dry cell battery was invented by Georges Leclanché in 1866, when he replaced the liquid electrolyte with a moist paste and enclosed the cell in a zinc container. This battery, called the Leclanché cell, was more convenient and portable than the previous wet cells, and was used for telegraphy and other purposes.
- The first nickel-cadmium (NiCd) battery, the first alkaline battery, and the first lithium battery were invented in the 20th century, by Waldemar Jungner, Lewis Urry, and M. Stanley Whittingham, respectively. These batteries offered higher energy density, longer cycle life, and better performance than the previous batteries, and enabled the development of many portable electronic devices .
- The current state-of-the-art battery is the lithium-ion (Li-ion) battery, which was developed in the 1980s and 1990s by John Goodenough, Akira Yoshino, and others.This battery uses a variety of materials, such as cobalt, nickel, manganese, and graphite, to store and release lithium ions between the electrodes. This battery has the highest energy density, power density, and efficiency of all batteries, and is widely used for smartphones, laptops, electric vehicles, and grid storage .
FAQs About Batteries
What are batteries?
Batteries are devices that store chemical energy and convert it into electrical energy when needed. Batteries consist of one or more electrochemical cells, which are the basic units of batteries. Each cell has a positive electrode (cathode), a negative electrode (anode), and an electrolyte that allows the flow of ions between the electrodes.
How do batteries work?
Batteries work by creating a chemical reaction between the cathode and the anode, which results in the transfer of electrons from the anode to the cathode through an external circuit. The electrons provide the electric current that powers the device connected to the battery. The electrolyte facilitates the movement of ions between the electrodes, which balances the charge in the cell. The chemical reaction continues until one or both of the electrodes run out of reactants, which limits the battery’s capacity and lifespan.
What are the types of batteries?
Batteries can be classified into two main types: primary and secondary. Primary batteries are disposable batteries that cannot be recharged, such as alkaline, zinc-carbon, and lithium batteries. Secondary batteries are rechargeable batteries that can be used multiple times, such as lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries.
What are indium batteries?
Indium batteries are a type of lithium-ion batteries that use indium as the anode material. Indium is a soft metal that can form a smooth and uniform layer of lithium during charging and discharging, avoiding the formation of dendrites that can degrade the battery performance and safety.
What are the advantages of indium batteries?
Indium batteries have several advantages over conventional lithium-ion batteries, such as:
- Faster charging: Indium batteries can charge in under five minutes, which can reduce the range anxiety of electric vehicle (EV) drivers and enable wider adoption of EVs.
- Longer cycle life: Indium batteries can maintain stable performance over extended cycles of charging and discharging, which can increase the lifespan and reliability of the battery.
- Higher energy density: Indium batteries can store more energy per unit mass and volume than conventional lithium-ion batteries, which can improve the performance and efficiency of the battery.
What are the challenges of indium batteries?
Indium batteries are still in the research and development stage, and there are some challenges that need to be overcome before they can be commercialized, such as:
- Cost: Indium is a relatively rare and expensive metal, which can increase the cost of indium batteries compared to conventional lithium-ion batteries.
- Scalability: Indium batteries require precise control of the indium layer thickness and morphology, which can be difficult to achieve at large scales and with different cathode materials.
- Stability: Indium batteries may suffer from side reactions with the electrolyte and the cathode, which can affect the stability and safety of the battery.





